Pharmaceutical, Medical Device and Combination Device Testing
Cormica Appoints Mark Botting as Chief Commercial Officer
Cormica is pleased to announce the appointment of Mark Botting as Chief Commercial Officer (CCO). Mark brings over 20 years of leadership experience in the contract research and laboratory testing sector, having held senior commercial and operational roles within several global life science organisations. Most recently, he led strategic development across a network of laboratories serving the pharmaceutical and medical device industries.
Mark’s extensive experience in supporting global healthcare manufacturers and deep understanding of regulatory testing requirements make him ideally suited to help drive Cormica’s next phase of growth. His track record in building high-performing commercial functions and delivering long-term client value is aligned with Cormica’s commitment to excellence.
As CCO, Mark will lead the development of Cormica’s commercial strategy and enhance the organisation’s ability to deliver tailored solutions that meet the evolving needs of clients. He will play a key role within the leadership team, focused on strengthening market presence and deepening relationships across the pharmaceutical, medical device and combination product sectors.
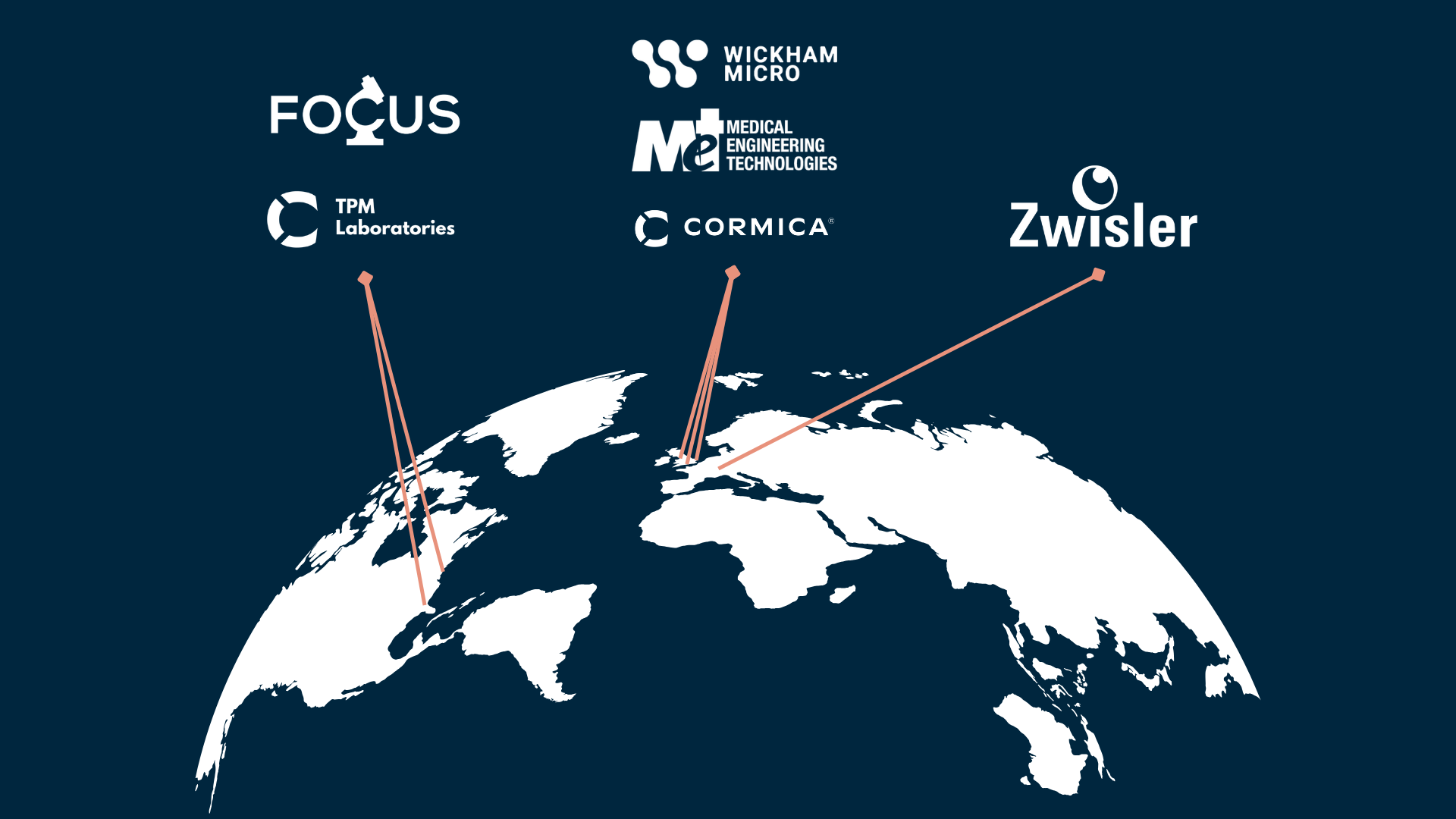
Cormica provides end-to-end testing solutions, from early development support through to post-market surveillance. With GMP, GLP and ISO 17025 accreditations, and facilities across the UK, EU and US, Cormica helps clients bring safe, effective and compliant products to market efficiently and confidently.
Mark joins Cormica with enthusiasm and a strong vision for sustainable commercial growth and customer-focused service delivery.
Commenting on the appointment, Mark Hammond, CEO of Cormica said:
“We are delighted to welcome Mark to the team. His experience and leadership will be invaluable as we enter the next phase of our growth journey. Mark’s strong background in the testing and CRO industry will help us deliver even greater value to our clients and reinforce our commitment to quality, compliance and innovation.”
About Cormica
Cormica Group (formerly known as WMP Group) is a medical device and pharmaceuticals testing group enabling life science manufacturers and med tech innovators to launch their products more quickly, more safely and to more markets. Limerston Capital LLP acquired Wickham Microbiology Ltd in September 2021 and Medical Engineering Technologies in May 2022, with Zwizler Laboratorium and Focus Labs both in January 2025, forming Cormica Group as the collective Group operates as today.
Contact Us
Ready to take the next step? We’d love to hear from you. Contact us today to discuss your testing needs and find out how Cormica can help you achieve your goals.

