Device & Transit Testing
Comprehensive Physical & Packaging Testing for Medical Devices & Combination Products
Accelerate your medical device’s path to market with Cormica’s rigorous Physical & Packaging Testing solutions. Our experts validate your device’s functionality, durability, packaging integrity, and safe transport, ensuring product performance, patient safety, and regulatory compliance throughout development and distribution.
Testing Excellence You Can Trust
Cormica’s Physical & Packaging Testing services are conducted in accordance with ISO 17025 standards. This accreditation underscores our dedication to reliable data, robust testing protocols, and compliance with industry-leading practices.
Request a call back from our team to learn how Cormica can safeguard your product’s success.
Your Partner in Device Validation & Packaging Verification
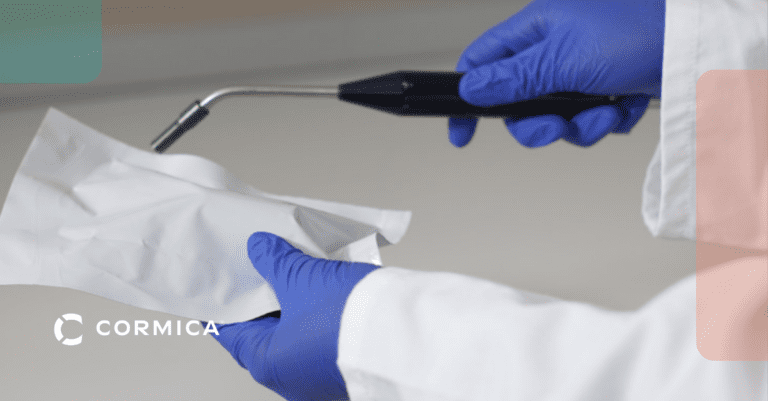
Device Performance & Safety Testing
-
Device Functionality Testing
Evaluates how devices respond to forces like tension, compression, and bending, ensuring they withstand stresses during use.
-
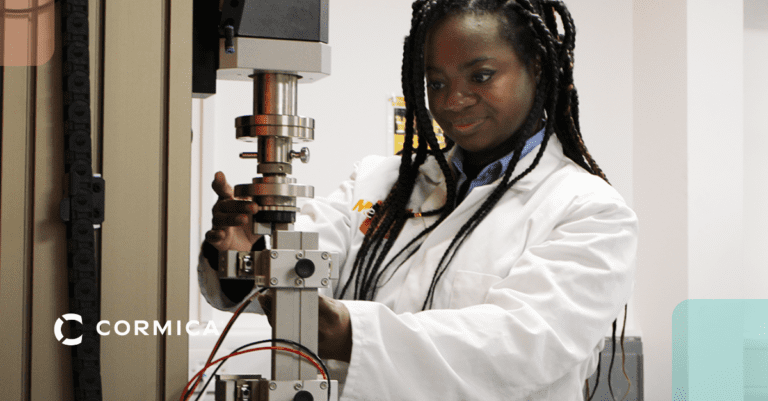
Device Durability Testing
Assesses how well your device withstands forces, wear and tear, and repeated use throughout its intended lifespan.
-
Device Sterilisation Compatibility
Verifies that your device materials and performance aren't compromised by sterilisation methods (heat, radiation, etc.).
-
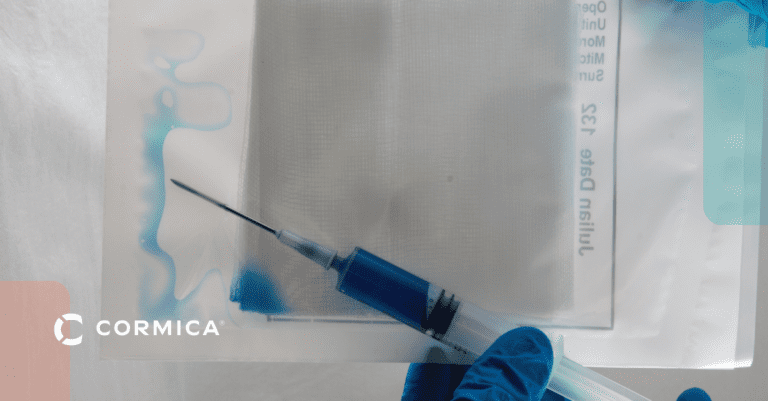
Drug-Device Interaction Testing (Combination Products)
Investigates how your drug formulation and device components interact, ensuring safety and effectiveness.
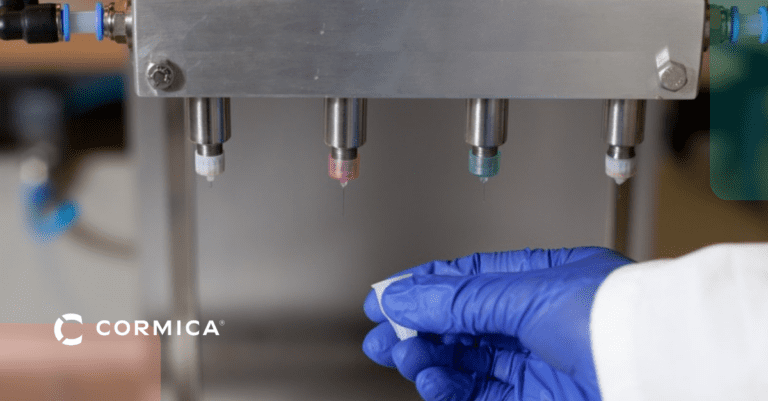
Packaging & Transit Performance Testing
-
Seal Strength & Integrity Testing
Ensures your medical device packaging remains tightly sealed to prevent contamination and product leakage.
-
Packaging Barrier Performance
Evaluates how well your packaging protects your device from moisture, light, oxygen, and other environmental factors that could affect product quality.
-
Packaging Durability Testing
Assesses your packaging's resilience against wear, tear, punctures, and environmental stresses throughout its shelf life, safeguarding your device's functionality and safety.
-
Transit Validation Testing
Replicates real-world shipping conditions (Drops, Vibration, Compression) to assess if your packaging safeguards your device effectively.
-
Regulatory-Aligned Testing
Ensures your device and packaging meet industry standards (ASTM D4169, ASTM D7386) for product safety and regulatory compliance.
Meeting Global Standards for Medical Device & Packaging Testing
Cormica’s Physical & Packaging Testing services adhere to the most stringent regulatory requirements and industry best practices, including:
Device Performance & Safety Testing Standards:
- ISO 11608 Series (Needle-based injection systems)
- ISO 11040 Series (Prefilled syringes)
- ISO 80369 Series (Small-bore connectors for liquids and gases)
- ISO 7864 (Sterile hypodermic needles)
- ISO 11607-1 and ISO 11607-2 (These critical standards focus on packaging for terminally sterilised medical devices.)
- EU Medical Device Regulation (MDR) (Ensures compliance with the MDR’s stringent requirements for safety, performance, and documentation throughout your device lifecycle.)
Packaging & Transit Performance Testing:
- ASTM D4169 (Performance Testing of Shipping Containers and Systems)
- ASTM D7386 (Performance Testing of Packages for Single Parcel Delivery Systems)
- ASTM F1886/F1886M (Visual Inspection for Flexible Packaging)
- ASTM F88/F88M (Seal Strength of Flexible Barrier Materials)
- ASTM F1140 / F2054 (Burst Testing of Flexible Package Seals)
- ASTM F1929 (Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration)











Why Cormica?
Cormica offers a unique combination of expertise, regulatory knowledge, and a commitment to client success, making us your ideal partner for Physical & Packaging Testing.
One-Stop Solution
From materials analysis and device functionality testing to packaging integrity and regulatory compliance, Cormica streamlines your entire testing process.
Medical Device Specialists
Our deep understanding of medical device regulations, standards, and unique requirements ensures testing that is tailored to your needs and accelerates your path to market.
Partnership Approach
We work collaboratively with you to develop customised testing plans, offering clear guidance and actionable insights to optimise your device and packaging performance.







