ISO 18562-3:2024 Carbonyl Testing Requirements and What Manufacturers Need to Know
Evolving requirements for breathing gas pathway testing place new pressure on manufacturers. Cormica provides the carbonyl testing expertise, method development and regulatory guidance needed to transition smoothly to ISO 18562-3:2024. We help you generate clear, compliant data that supports faster submissions and reduces costly project delays.
From 5 July 2026, the U.S. Food and Drug Administration (FDA) will no longer accept submissions based on ISO 18562-3:2017 for breathing gas pathway testing. Study data must comply with the updated ISO 18562-3:2024 requirements, including specific assessment of formaldehyde and other carbonyl compounds. Cormica has implemented the required methodology so manufacturers can align with the latest expectations without delays to market access.

Authors:

James Silk
Senior Analytical Chemist at Cormica, specialises in extractables and leachables (E&L) testing for medical devices and combination products. With expertise in GC-MS and LC-MS techniques, James supports regulatory submissions through method development, validation, and interpretation of E&L data for global clients.
Jackie Waller
Senior Analytical Chemist at Cormica MET.
Share with your colleagues:
What Has Changed in ISO 18562-3:2024?
The latest edition of ISO 18562-3 extends its normative references beyond ISO 16000-6 to now include parts 3 and 4 as well. This change makes the specific assessment of formaldehyde and other carbonyl compounds compulsory, rather than optional.
From 5 July 2026, any new test study submitted to the FDA for breathing gas pathways must be based on ISO 18562-3:2024, not the 2017 version. Manufacturers therefore need analytical methods that can robustly quantify relevant carbonyl compounds in volatile organic substance (VOS) testing.
Why Monitor Carbonyl Emissions From Breathing Gas Pathways?
Many carbonyl compounds are recognised as hazardous and can:
- Irritate mucous membranes
- Contribute to carcinogenic risk
- Potentially impact the nervous system over the long term
For medical devices that deliver gas directly to the lungs, any emitted volatile or semi-volatile carbonyl compounds can be inhaled immediately. Because breathing gas pathways bypass some of the body’s natural protective filters, there is a higher exposure risk if these compounds are not understood and controlled.
How Medical Devices Can Release Carbonyl Compounds
The presence and release of carbonyl compounds from device materials may be associated with:
- Residues from sterilisation processes
- Polymer degradation over time
- Evaporation of adhesives and solvents
Their volatile nature, combined with airflow through the device, can promote release of these compounds into the gas pathway. Elevated temperatures, such as those found in temperature-controlled incubators, may further increase emission levels.
Continuous monitoring of carbonyl emissions helps ensure by-products are identified and mitigated early, supporting both patient safety and regulatory compliance.

Devices and Systems Affected by ISO 18562-3 Carbonyl Testing
A breathing gas delivery system typically consists of multiple interconnected components, each with its own potential for VOS and carbonyl release, such as:
- Ventilators and respiratory support systems
- Breathing tubes and circuits
- In-line filters and humidifiers
- Masks and interfaces
- Incubators and other temperature-controlled gas delivery systems
Testing the complete system under clinically relevant conditions provides a more realistic assessment of the total patient exposure.
Cormica’s analytical approach to ISO 18562-3:2024 carbonyl testing
Cormica has incorporated specific carbonyl compound analysis, including formaldehyde, into its established VOS testing methods for breathing gas pathways.
We simulate the clinical use of devices designed to deliver gas to a patient, so that the gas delivery system can be evaluated in its entirety. This approach promotes realistic VOS release, including relevant carbonyl compounds, under exaggerated but clinically relevant test conditions.
Capturing carbonyls using DNPH derivatisation
Because carbonyl compounds such as formaldehyde are small and highly volatile, traditional VOS capture techniques can lead to losses during sampling. This reduces analytical efficiency and can compromise data quality. Gas chromatography may also be challenging due to limited retention on the column.
To overcome this, Cormica uses derivatisation at the point of release, based on the well-known reaction between aldehydes/ketones and 2,4-dinitrophenylhydrazine (DNPH):
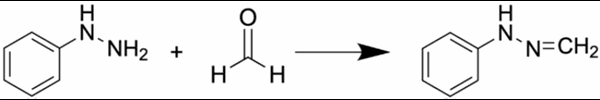
By installing DNPH-containing cartridges within the gas pathway beyond the medical device in a simulated test set-up, any released carbonyl compounds are captured and converted into their DNPH-derivatised forms. These derivatives are more stable and suitable for sensitive chromatographic analysis.
LC-UV method performance for DNPH-derivatised carbonyls
Using an Agilent 1290 Infinity II LC-UV system, Cormica has developed a method capable of fully resolving and detecting 13 DNPH-derivatised carbonyl compounds.
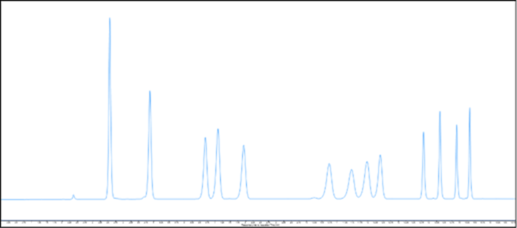
This method supports confident assessment of carbonyl compounds typically between C1 and C12. The enhanced sensitivity of the system allows detection of concentrations as low as 10 parts per billion (ppb), enabling detection of compounds released from a wide range of materials used in medical device manufacture.

Preliminary validation data demonstrate:
- Linear range from 50 ppb to 20 ppm for all 13 carbonyl compounds
- Pearson correlation coefficient > 0.99 for all compounds
- Relative standard deviation (%RSD) < 1 % for all compounds
- Limit of detection (LOD) of 10 ppb (signal-to-noise ratio ≥ 3)
- Limit of quantitation (LOQ) defined as three times the LOD
This analytical performance provides a robust basis for regulatory submissions and supports greater confidence in understanding carbonyl emissions from breathing gas pathways.

Supporting ISO 18562-3:2024 compliance and patient safety
By integrating specific carbonyl testing into its VOS methodology, Cormica helps manufacturers:
- Align with the updated requirements of ISO 18562-3:2024
- Generate data suitable for FDA submissions beyond July 2026
- Understand carbonyl contributions from each device component and the complete system
- Strengthen risk management for breathing gas pathways and related devices
Talk to us about ISO 18562-3:2024 testing
If you are developing or updating devices with breathing gas pathways and need to understand how ISO 18562-3:2024 impacts your testing strategy, Cormica can help.
Other Cormica News, Events & Resources
Cormica's Webinars
Recent News
Technical Resources
Laboratories near you, supporting global product success
With accredited laboratories in the UK, EU and US, we support pharmaceutical, medical device and combination product manufacturers worldwide. Local support combined with global quality, delivered by teams who understand your regulatory requirements.
Our Clients’ Experiences




