ISO 18562: 2024 - Biocompatibility evaluation of breathing gas pathways in healthcare applications
Type:
Published:
Authors:
Luminita Moraru – MSc | MRSC | MBTS
Analytical Chemistry Manager
Medical Engineering Technologies
Elena Henderson – BSc | MBSI | MRSB | MBTS
Biocompatibility Assessor
Medical Engineering Technologies
Request More Information
First released in 2017, ISO 18562: Biocompatibility evaluation of breathing medical gas pathway in healthcare applications series addresses the biological evaluation of gas pathway medical devices.
The long waited ISO 18562 has now finally been updated and Medical Engineering Technologies has released an article highlighting the changes and their impact on new products for manufactures as well as testing labs.
Read the article bellow to understand the changes and how those could affect the route of proving biocompatibility of your device.
Gas pathway are the interior surfaces which gases or liquids pass that can be inspired as per the newest version ISO 18562-1:2024.
The change starts from the definition of gas pathway in the new version when comparing to the 2017 version, where gas pathway was defined as interior surfaces, over which gases or liquids, in a MEDICAL DEVICE bounded by the ports through which gases or liquids enter and leave the MEDICAL DEVICE including the PATIENT interface or the interior surfaces of enclosures that are in contact with gases or liquids that can be inspired.
ISO 18562-2024: Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 1:Evaluation and testing within a risk management process
Multiple new definitions and terms have now been added to improve the biocompatibility of gas pathway medical devices, such volatile organic substances instead of volatile organic compounds as in previous 2017 version.
The latter 2024 version of the standard defines volatile organic substance VOS as any organic substance with a saturation pressure at standard temperature of 20 °C that exceeds current analytical detection threshold. The VOS now include VOCs, SVOCs and VVOCs and the evaluation is not only focusing on volatiles only.
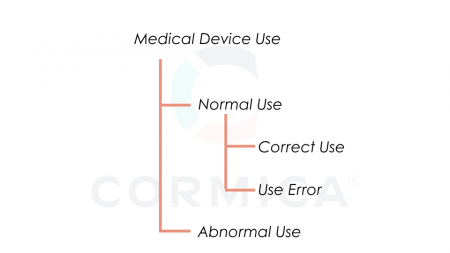
A new term in the standard is abnormal use and is defined conscious, deliberate act or deliberate omission of an act that is counter to or violates normal use and is also beyond any further reasonable means of user interface-related risk control by the manufacturer, bellow is relationship between the types of use for a medical device as per ISO 18562-1:2024.

Patient groups have been expanded to 6 categories and it is worth pointing out that the neonate, infant and paediatric weight of has now wider range and on contrary the adult weight has lower limit of 56 kg as opposed to 70kg.
| Patient group according to ISO 18562-1 2017 | Patient group according to ISO 18562-1 2024 | ||
| Neonate | 0,5 kg | Premature neonate | <2,65 kg |
| Infant | 3,5 kg | Infant 0- 0.5 yr | 2,65-7,4 kg |
| Small child 0,5-3yr | 7,4-15 kg | ||
| Paediatric | 10kg | Child 3-10yr | 15-32 kg |
| Adolescent 10-18yr | 32-56 kg | ||
| Adult | 70kg | Adult >18yr | >56 kg |
On this note, new terms and guidance on resting breath rate, and default daily breathing volume when resting have been introduced. These new values and definitions will have an impact in the further testing to be performed, if deemed necessary such as to estimate the inhalation dose to the patient for the tests of ISO 18562-3, convert the concentration of each substance to a total dose per patient per day based on the breathed volume.
| Patient group | Label | Typical age range | Ideal body mass range (kg) | LPV (ml/kg) | Resting breath rate (breaths/min) | Default body mass (kg) | Default daily breathing volume (resting) (m3/d) | Maximum exercise ventilation (m3/h) |
|---|---|---|---|---|---|---|---|---|
| Premature | neonate | Premature birth | <2.65 | 6 | 60 | 0.5 | 0.26 | — |
| Infant | 0 to 0.5 | 2.65 to 7.4 | 8 | 56 | 44 | 3.5 | 2.3 | — |
| Small child | 0.5 to 3 | 7.4 to 15 | 8 | 44 | 26 | 5.1 | 6.0 | 1.8 |
| Child | 3 to 10 | 15 to 32 | 8 | 26 | 20 | 20 | 7.7 | 2.6 |
| Adolescent | 10 to 18 | 32 to 56 | 8 | 21 | 32 | 7.7 | 11.5 | 4.6 |
| Adult | >18 | >56 | 8 | 20 | 60 | 11.5 | 4.6 | — |
Single transitory contact duration with accumulative limited exposure has now been included into the consideration and with their evaluation can be completed with good rationale. For example: Single use inhalers, respiratory gas monitors, mouth pieces and masks would not require testing with the justification given, however thorough review of the coatings applied to those will have to be evaluated.
The new important Annex ZA has been added, where the relationship between International standard and European Standard edition as a presumption of conformity has been established.
The risk management process has changed to more detailed version which supports the risk management process not only with detailed aspects and conditions of the studies. It also includes the weight of the rationale in this process which allows to avoid unnecessary tests that would provide no value to the risk evaluation.
It is also now clearly stated that the same risk evaluation standard will be used throughout the ISO 18562 the same as used within ISO 10993 series.
The diagram assessing the risk of use of a breathing medical device has been updated to a more comprehensive version including step by step the process to follow when addressing biocompatibility of a gas pathway medical device, see bellow new version as per 2024 standard.
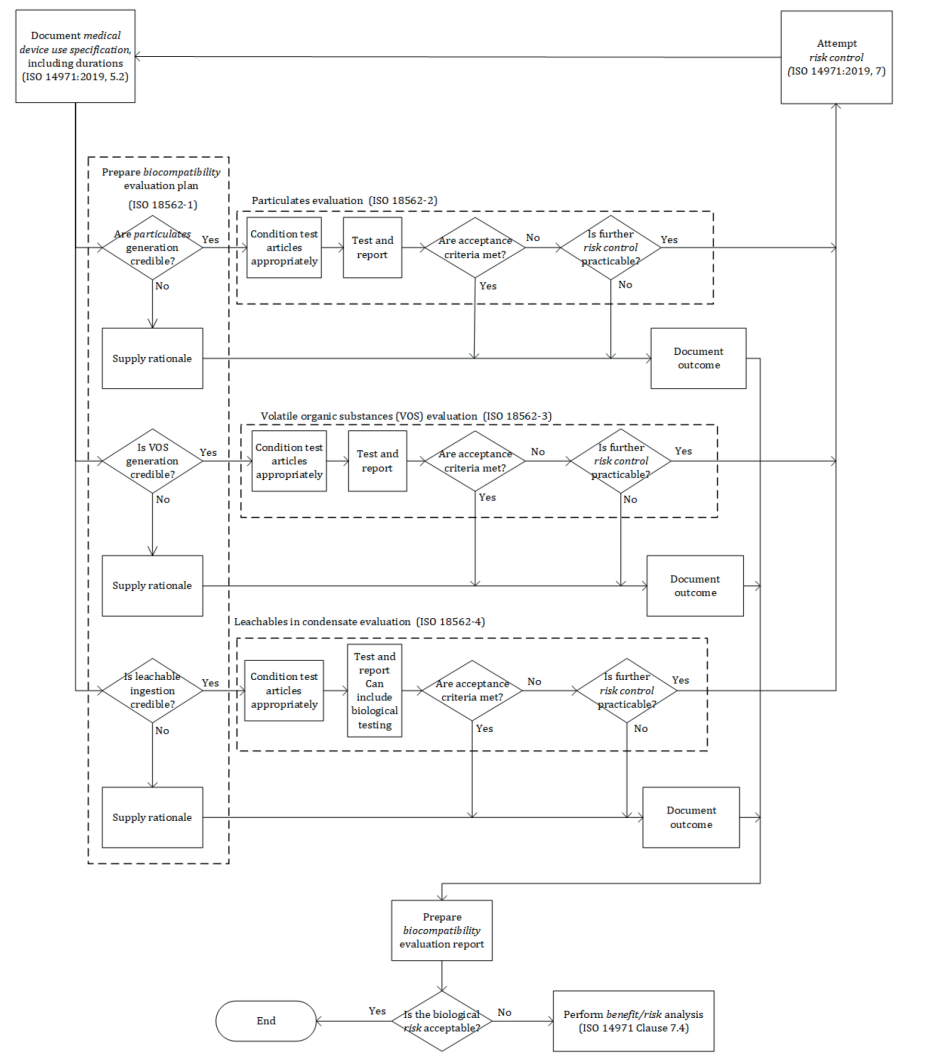
Although the series do not address biocompatibility hazards associated with inorganic gases, the 2024 version of ISO 18562-1 mentioned that, when applicable and some authorities having jurisdiction require the manufacturer to evaluate the following:
— Ozone, for gas pathways in contact with active electromechanical or electrostatic parts in normal condition;
— CO, NOx and CO2, for gas pathways where inorganic gases are added, generated or concentrated;
ISO 18562-2024: Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 2: Tests for emissions of particulate matter
It is now clearly stated that medical devices containing water tanks shall be evaluated for particulate emissions without the water.
While the acceptance criteria and suggested methods for the particulate emission evaluation remains the same:
– Less than or equal to 2,5 µm diameter, in excess of 12 µg/m³;
– Less than or equal to 10 µm diameter, in excess of 150 µg/m³.
The range of particulates evaluated has now changed from addressing particulates with a diameter of 0.25µm 0.20 µm, as per the previous version.




ISO 18562:2024 Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 3: Tests for emissions of volatile organic substances
While the new version states that the evaluation of volatile organic substances should be performed at a time after manufacture that represents the shortest reasonable time that could elapse between manufacture and use with a patient. The worse case scenarios for the testing is still recognised as being the highest clinically relevant ambient temperature until the medical device has achieved thermal stability. The use of the highest clinically relevant temperature to facilitate faster or accelerated testing.
However, care is to be taken to ensure that the high temperatures will not alter the chemical composition of the VOS emitted.
When it comes to worse case simulation of the clinical use of the medical device to evaluate the level of volatile organic substances emitted the lowest usable setting for delivery to the patient considering the volumes and pressures.
Target compounds analysis has now been introduced in the scope of the study and those have specific limit of reporting, this equals 1µg/m³ while the non-target compounds limit of reporting remains as per the previous version at 2 µg/m³.
The risk associated with the release of carbonyl groups is now evaluated as part is ISO 18562-3, while formaldehyde assessment is included.
As the Volatile Organic Compounds have now been replaced by Volatile Organic Substances including a higher range of compounds with different boiling points, this is clearly explained in table bellow (ISO 18562-3:2024):
| Description | Abbreviation | Boiling point range °C | Example compounds |
|---|---|---|---|
| Very volatile organic compounds | VVOC | < 50 °C | Propane, butane, formaldehyde, methyl chloride, methylene chloride |
| Volatile organic compounds | VOC | 50 °C to < 250 °C | d-Limonene, toluene, acetone, ethanol (ethyl alcohol), 2-propanol (isopropyl alcohol), hexanal |
| Semi-volatile organic compounds | SVOC | 250 °C to 400 °C | Pesticides (DDT, chlordane), plasticizers (phthalates), fire retardants (PCBs, PBB) |
ISO 18562-3:2024- Table A.1 — Classification of volatile organic substances
ISO 18562-2024: Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 4: Tests for leachables in condensate
The scope of the 2024 version of the standard has mainly remained the same, with the intend to protect patients connected to medical devices from harmful amounts of substances that might be dissolved in water that has condensed in the gas pathways of those medical devices. The information provided applies over the expected lifetime of the medical device in use, however it does not address biological evaluation of the surfaces of as pathways that have direct contact with the patient.
New statement added in the 2024 versions explain that If the experimentally determined volume of condensate reaching the patient establishes that no more than 0,1 ml in 24 h reaches the patient under worst case clinical conditions, then no further testing is required which will help the manufacturer and the biocompatibility assessor establish prior to any testing.
The leachable components can equally cause the irritation of the patient respiratory organs and the tissues of the mucosal pathways. Biocompatibility plan for breathing gas pathways now will consider Cytotoxic, Irritational and Sensitizing effects of the potential leachable from the condensate, whereas in the past only Cytotoxic and Sensitising responses were reviewed. This addition in testing has been part of the new ISO 18562-4:2024 revision.
This also harmonises, in terms of local tissue responses, with ISO 10993 series.
As part of the chemical characterisation of the condensate organic leachable substances has been introduces. Those substances should be identified and quantified using:
- Gas chromatography-mass spectrometry (GC/MS); or
- Liquid chromatography – mass spectrometry (LC/MS).
- Elemental (e.g. metal ions) leachable substances, using appropriate analytical techniques such as
- Inductively coupled plasma/mass spectroscopy (ICP-MS); or
- Inductively coupled plasma atomic emission spectroscopy (ICP-AES); or
- A combination of ICP-MS and ICP-AES.
The scope of the study has expanded in comparison to the previous version where only semi-volatile organic compounds analysed by GCMS and elemental analysis via ICP was mentioned.
The metal ions analysis is now performed according to ICH Q3D not USP 233 as per previous version.
Exaggerated extraction is an addition to the 2017 version of the standard as part of the sample collection technique. While in the previous version the focus was on simulating the clinically relevant conditions, in the new standard the worse-case extractions must be considered. The definition of the exaggerated extraction is extraction that is intended to result in a greater amount of a chemical constituent being released as compared to the amount generated under the simulated conditions of use with a note mentioning that It is important to ensure that the exaggerated extraction does not result in a chemical change of the material.
The materials released during the studies performed as per either part 2, 3 or 4 of ISO 18562:2024 standards must be risk assessed by a toxicologist to highlight any concerns that may occur due to the use of the medical device in clinical conditions.
For the leachables released as per part 4 the estimation of the exposure dose to the patient shall be made by converting the concentration of each substance to a total dose per patient per day based on the condensate volume and assume the total quantity is released each day.
The exposure dose of each identified substance is compared to its specific tolerable intake.
If no toxicological data available then the threshold of toxicological concern is used as per ISO 18562-1:2024:
| Total exposure period | TTC value μg/d |
|---|---|
| ≤ 1 month | 120 |
| > 1 month to ≤ 1 year | 20 |
| >1 year to ≤ 10 years | 10 |
| > 10 years | 1,5a |
| a The 1,5 μg/d value is based on 10-5 cancer risk and 60 kg (adult) body mass[22][29]. | |
ISO 18562-1:2024 – Table 2 — TTC values by total exposure period
When the exposure dose of an identified substance delivered to the patient is more than the tolerable intake or TTC the MOS (margin of Safety) is calculated, which in this case will equal the tolerable intake by the exposure dose.
The limits of the MOS stated in part 4 are as bellow:
- Consider whether the risks indicated by such margin of safety values (i.e. <1) can be addressed.
- Conduct a benefit-risk analysis utilizing ISO 18562-1:—, Clause 10.
The new changes in the standard regulations has a huge impact on the requirements of gas pathway medical devices and all labs are now adapting to the changes to address and meet the manufacturers expectations to endure minimal delays in submissions.
We are up to date with the changes and can work with medical device manufacturers to adapt the new requirements and meet your deadlines – Contact us to see how we can help you.