QC Batch Release Testing
Home » Services » Analytical Chemistry » QC Batch Release Testing
QC batch release testing is a regulatory requirement under Good Manufacturing Practice (GMP), ensuring every batch of a pharmaceutical, medical device, or combination product meets predefined specifications before it reaches patients.
Without compliant release data, products cannot legally be placed on the market. As global regulatory expectations tighten, accurate and timely final product release testing is essential to support submissions, mitigate risk, and maintain supply chain continuity.
Our analytical services support a wide range of sectors including pharmaceuticals, medical devices, advanced wound care, healthcare, and agrochemicals.
Cormica’s Expertise
Cormica provides GMP batch release testing from our global laboratories. Our unique blend of spectroscopic, chromatographic, solid state and elemental analysis backed with over 30 years of analytical chemistry experience and 60 years scientific heritage, allow us to deliver compliant, audit-ready results with speed and precision.
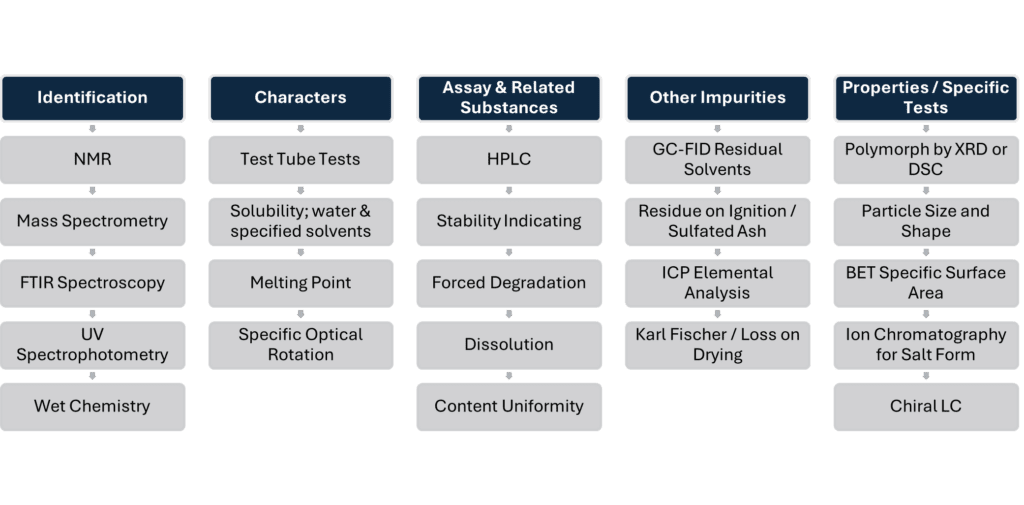
What We Test
Our QC batch release services cover:
- Raw materials and APIs
- Finished product testing – tablets, injectables, creams, devices
- Combination product batch release
- Veterinary and advanced wound care products
- Complex formulations and non-compendial matrices
We offer testing via client methods, pharmacopoeial methods (USP, Ph. Eur.), or bespoke method development and validation.
Quality
- MHRA GMP Accredited
- MHRA GLP Accredited
- FDA Inspected
- ISO 9001:2015
QC Service Offering
- Method Development
- Method Establishment
- Method Verification / Validation
- QC Batch Release
- ICH Stability Storage & Testing
Sample Types
- Raw Materials
- Critical Excipients
- Active Ingredients / Drug Substance
- Formulations
- Finished Products / Drug Products
- Wide Range of Dosage Forms
Method Types
- Limit Tests
- Quantiative Methods
- Client Methods
- Monograph / Literature Methods
- Bespoke Methods

Using our extensive range of analytical equipment, we offer QC batch release testing services to both the human and veterinary pharmaceutical sectors, plus advanced wound management, medical devices and combination products.
Our Capabilities
- GMP – compliant quality control testing
- Expert Led Protocol Design & Study Management
- HPLC, GC, UV, FTIR, Karl Fischer, ICP-MS
- Method transfer, verification, and validation
- Support for regulatory submissions (CTD Module 3)
- Seamless integration with microbiology and physical testing teams
Development Support to Post-Market Surveillance
We work closely with our Microbiological QC and ICH Stability teams to provide complete support from development to final product release helping you reduce delays, ensure regulatory compliance, and maintain product quality across the lifecycle.
Contact Us
Let’s ensure your products are released safely, quickly, and in full compliance.
